How to measure CO2?
De Beer-Lambert Law
Pierre Bouguer (1698 - 1758) observed, while looking at red wine, that the light entering the red wine was weakened. The more red wine the light has to pass through, the greater the attenuation. But the more concentrated the red wine, the greater the attenuation.
Although Pierre Bouguer discovered it, the law is often attributed to Johann Heinrich Lambert (1728-1777) because he referred to it in his book Photometria from 1760. In 1852 August Beer (1825 - 1863) discovered another weakening relation so that the law finally became the Beer-Lambert law (but sometimes also called the Lambert-Beer law or the Beer-Lambert-Bouguer law).
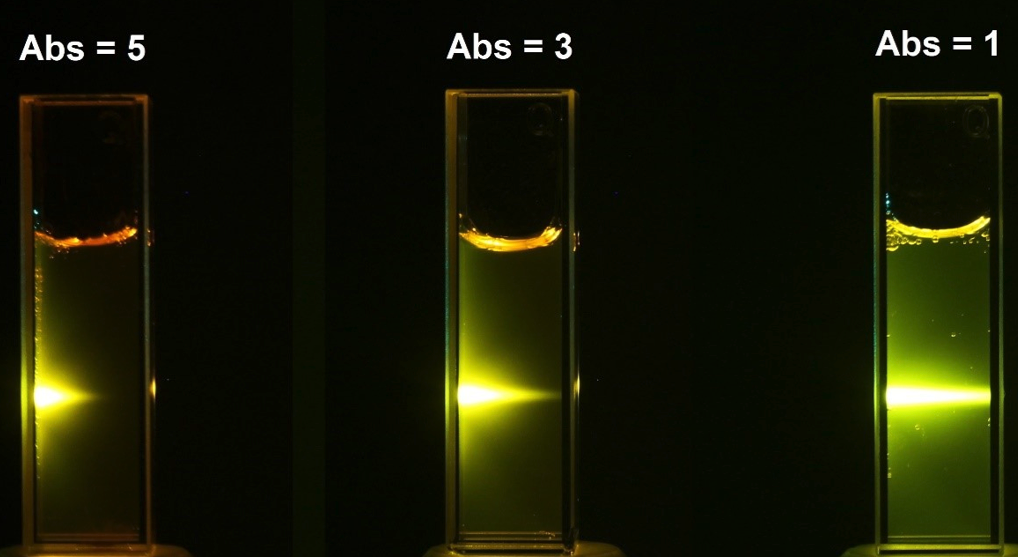
The Beer-Lambert law can be "easily" demonstrated with a green laser light in a solution of Rhodamine 6B. In the pictures below you can see that the light beam becomes weaker as it passes through more liquid. The liquid absorbs the light.


The figure below shows the same but with three different concentrations. The higher the concentration, the harder it is for the light to get through (more absorption of the light).
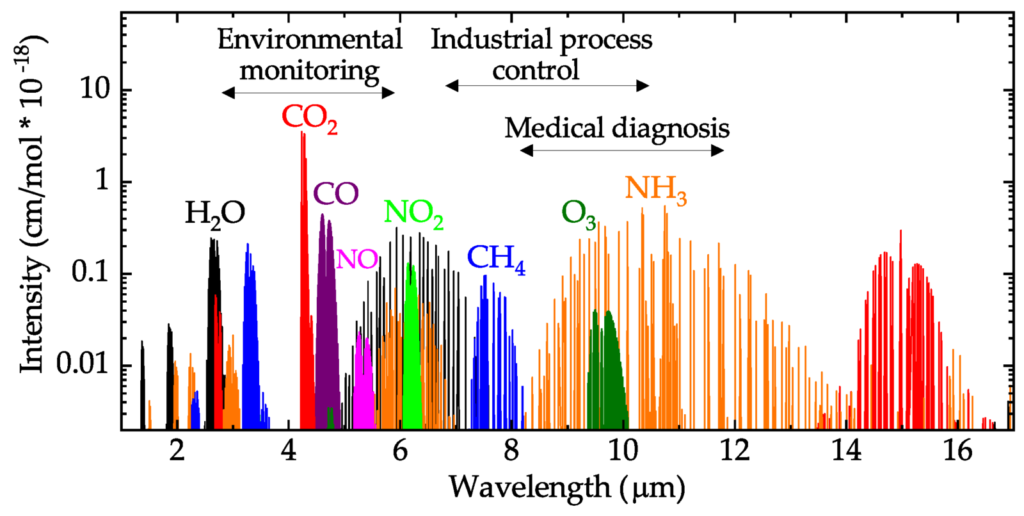
A similar absorption happens with gases. Only we have to use light that we cannot see.
In the figure below you can see that light with a wavelength of 4.26 um is absorbed by CO2 (for your information: visible light is between 0.38 and 0.7 um so we cannot see this).
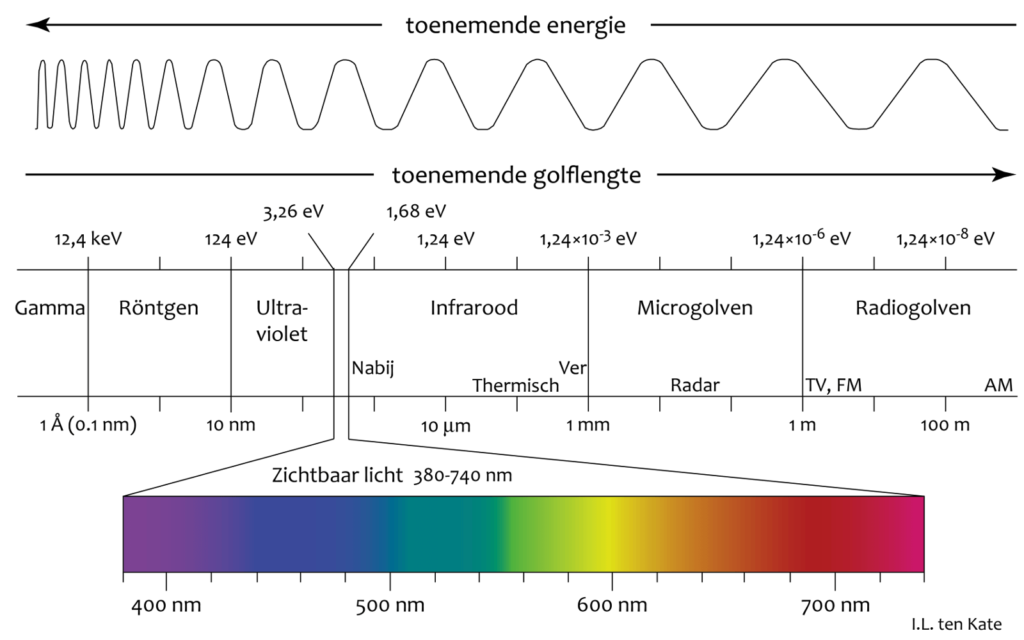
In the following figure you can see that the infrared part is between 0.7 um and 1 mm (1000 um) wavelength.
CO2 sensor measuring principle
With the above explanation, we have made a good start. We can measure CO2 by means of light. It is true that light is invisible to humans.
By shining light (infrared) on a gas (in our case the air) and seeing how much of this light is received on the other side, we can determine how much CO2 is in the air. The more CO2, the more the infrared light will be absorbed, and thus the less infrared light we receive. So the loss of light is a measure of the amount of CO2.
Below is a simple representation of a CO2 sensor. The most important parts are:
- Infrared lamp
- sample chamber or light tube
- light filter
- infrared detector
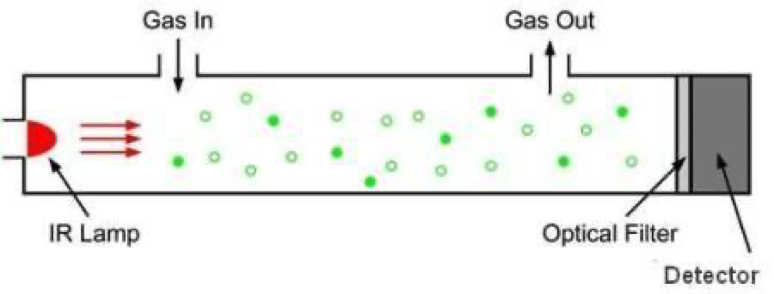
The infrared light is passed through the sample chamber to the detector. The CO2 in the sample chamber will absorb the infrared light so that not all the light reaches the detector. The less light that arrives, the more CO2 that is present.
What is the optical filter doing there?
The light source is not ideal. It does not exactly emit a light of 4.26 um. Also, the detector will not be perfect. It will not only respond to 4.26 um light. The light source will also send out other light (which may or may not be absorbed by other gases in the air) to which the light sensor will react. However, this reaction is not correct because it is not CO2. You might read out a wrong CO2 value. The optical filter solves this. This filter will only let through light of 4.26 um. So even if the infrared light is not perfect, the filter will stop this light. And even if the detector is not perfect, the filter will make sure that only the right light gets through. Making a "perfect" optical filter is also easier than making a "perfect" light source or light detector.
Usually the entrance and exit of the sample chamber are closed with a membrane. On our sensors, these are the white squares. This is to prevent dirt, dust or moisture from getting into the sample chamber.
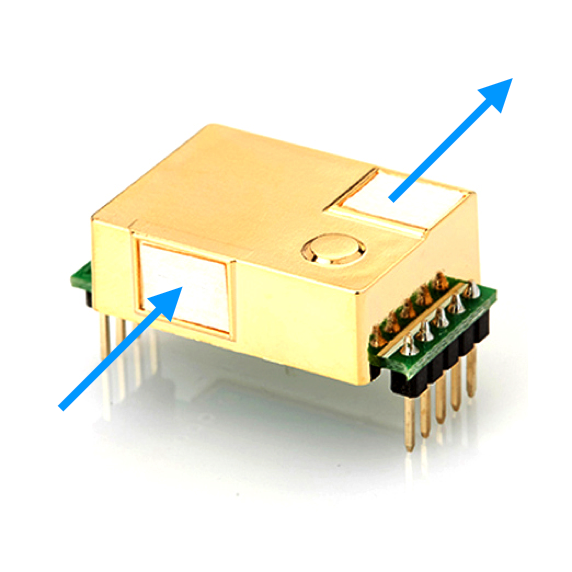
Calibration
So we measure how much light falls on the detector to determine how much CO2 is present. But what if the lamp starts to burn less? Surely every lamp will emit less light as it ages? Will our detector then give a wrong value?
Yes, this is possible and the only way to solve this is by calibrating the CO2 sensor. Calibration means putting the sensor in a known environment. Where you know exactly how much CO2 is present. Typically this is 400 ppm. Once you are in this known environment, you read out the sensor. You expect the sensor to give a value of 400 ppm. But suppose that it gives a value of 450 ppm. This could be a sign that the light source is weakened and the detector is receiving less light. This is not because there is 450 ppm of CO2. The detector is still working correctly but it is simply receiving less light because the light source has been weakened.
To compensate for the weaker light source, we calibrate. From the measurement above (450 ppm value measured but the environment is 400 ppm) we conclude that the ppm value given by our sensor is always 50 ppm too high. So if our sensor reads 450 ppm, we subtract 50 ppm and we get the real value. If our sensor reads 800 ppm, we also subtract 50 ppm and we know that the real value is 750 ppm. We have compensated for the weakened light source.
All this is of course corrected internally, in the sensor. The value read out on the display is therefore already the corrected value.
Do we need to calibrate our CO2 sensor now?
No, the CO2 sensor does this automatically. The CO2 sensor measures 24 hours a day, and always records the lowest value it measures. After one week, it takes the lowest value and sets it at 400 ppm.
Why?
The sensor assumes that during one week there was at least one moment when no one was present in the room (in schools, we may assume that no one is present at night or during the weekend). In an empty room, the CO2 value should be equal to 400 ppm. So if the sensor measured 450 ppm as the lowest value that week, it will assume that it is systematically measuring 50 ppm too high and will automatically correct internally.
Below is a CO2 measurement for 24 hours. Note that during the night the value drops to 400 ppm.
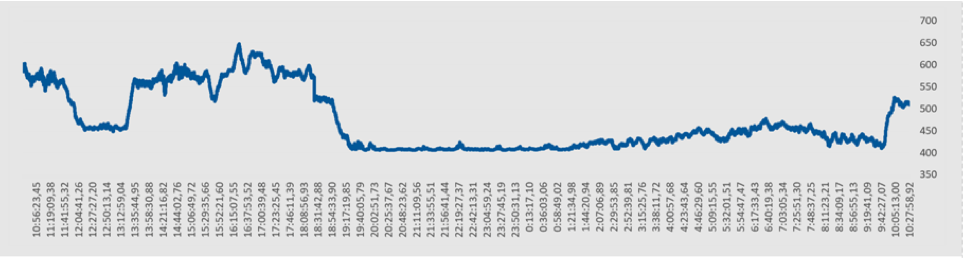
The above principle obviously only works if there is a regular situation where the CO2 ppm value can drop sufficiently. For example, in environments where people are constantly present (also at night) or in environments where the CO2 value hardly ever drops to 400 ppm (e.g. an underground car park), other CO2 sensors should be used. Which take this into account. Or the sensors must be manually calibrated on a regular basis.
